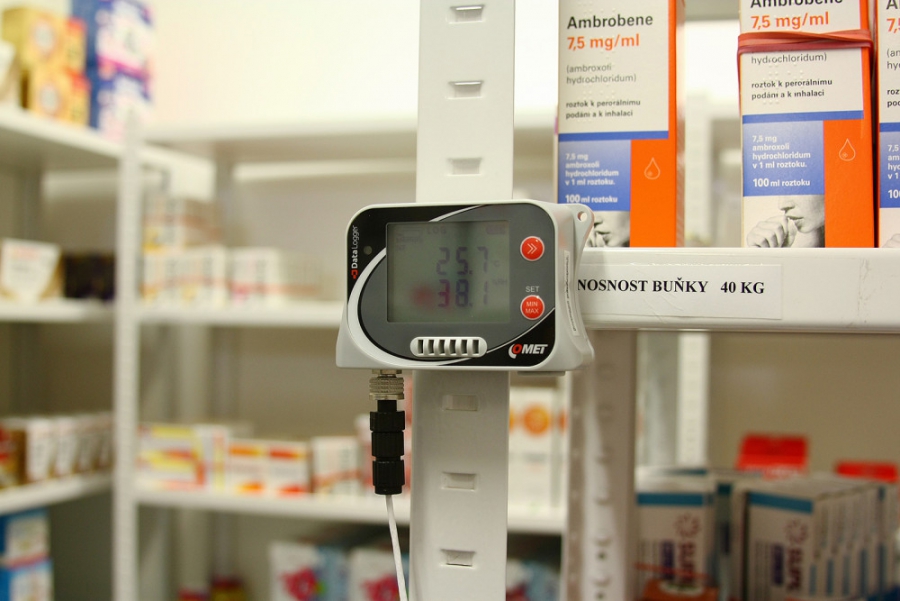
Temperature And Humidity In Pharmacy . the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. The who expert committee on specifi cations. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. who good practices for pharmaceutical microbiology laboratories.
from www.cometsystem.com
who good practices for pharmaceutical microbiology laboratories. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. The who expert committee on specifi cations. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room.
Temperature and Relative Humidity measuring in PHARMACIES and DRUG STORES
Temperature And Humidity In Pharmacy the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a.
From www.infectioncontrolresults.com
Temperature and Humidity Monitoring in Sterile Processing Infection Temperature And Humidity In Pharmacy the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. storage unit temperature/humidity distribution the range and pattern of temperatures and/or. Temperature And Humidity In Pharmacy.
From www.cometsystem.com
Temperature and Relative Humidity measuring in PHARMACIES and DRUG STORES Temperature And Humidity In Pharmacy the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi. Temperature And Humidity In Pharmacy.
From engineerexcel.com
Temperature and Humidity Relationship [+ Chart] EngineerExcel Temperature And Humidity In Pharmacy temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. the exact conditions should be defined by the product, but this. Temperature And Humidity In Pharmacy.
From www.temperaturehumidity-datalogger.com
Plastic Temperature And Humidity Data Logger For Medical Warehousing Temperature And Humidity In Pharmacy The who expert committee on specifi cations. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. the world health organization states that the ideal relative. Temperature And Humidity In Pharmacy.
From pharmacyscope.com
How can relative humidity be determined? Pharmacy Scope Temperature And Humidity In Pharmacy The who expert committee on specifi cations. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. who good practices for pharmaceutical microbiology laboratories. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. storage unit. Temperature And Humidity In Pharmacy.
From templates.rjuuc.edu.np
Printable Temperature And Humidity Log Template Temperature And Humidity In Pharmacy the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. who good practices for pharmaceutical microbiology laboratories. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. storage unit temperature/humidity distribution the range. Temperature And Humidity In Pharmacy.
From radiobridge.com
Pharmacies Use Wireless Temperature Sensors for Drug Storage Temperature And Humidity In Pharmacy temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. the world health organization states that the ideal relative humidity (rh) for. Temperature And Humidity In Pharmacy.
From www.cometsystem.com
Temperature and Relative Humidity measuring in PHARMACIES and DRUG STORES Temperature And Humidity In Pharmacy temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. . Temperature And Humidity In Pharmacy.
From pharmajobswalkin.com
SOP For Temperature, Relative Humidity, And Differential Pressure Temperature And Humidity In Pharmacy understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. the world health organization states that the ideal relative humidity (rh) for pharmaceutical. Temperature And Humidity In Pharmacy.
From www.smarttag.tech
Product V2 Smart Tag Inc. Temperature And Humidity In Pharmacy understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. The who expert committee on specifi cations. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. the environmental condition during pharmaceutical business is defined by. Temperature And Humidity In Pharmacy.
From www.pricepulse.app
Elitech Digital Temperature Humidity Data Logger Pharmacy Refrigerator Temperature And Humidity In Pharmacy the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. The who expert. Temperature And Humidity In Pharmacy.
From www.pricepulse.app
Elitech Digital Temperature Humidity Data Logger Pharmacy Refrigerator Temperature And Humidity In Pharmacy who good practices for pharmaceutical microbiology laboratories. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. temperature, relative humidity and ventilation should be appropriate. Temperature And Humidity In Pharmacy.
From pharmacyscope.com
Dehumidification Pharmacy Scope Temperature And Humidity In Pharmacy temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. The who expert committee on specifi cations. the environmental condition during pharmaceutical business is defined. Temperature And Humidity In Pharmacy.
From www.cometsystem.com
Temperature and Relative Humidity measuring in PHARMACIES and DRUG STORES Temperature And Humidity In Pharmacy temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. who good practices for pharmaceutical microbiology laboratories. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure. the world health. Temperature And Humidity In Pharmacy.
From www.researchgate.net
Humidity of five pharmacy along 16 days (zigzag line) compared with Temperature And Humidity In Pharmacy The who expert committee on specifi cations. the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. storage unit. Temperature And Humidity In Pharmacy.
From www.cometsystem.com
Temperature and Relative Humidity measuring in PHARMACIES and DRUG STORES Temperature And Humidity In Pharmacy the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. the environmental condition during pharmaceutical business is defined by temperature (t) and relative humidity. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. storage unit temperature/humidity distribution the range and pattern of. Temperature And Humidity In Pharmacy.
From www.scribd.com
ISPE Article Temperature and Humidity Monitoring Require in Pharma Temperature And Humidity In Pharmacy The who expert committee on specifi cations. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room. who good practices for pharmaceutical microbiology laboratories. temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of. . Temperature And Humidity In Pharmacy.
From can.grandado.com
pharmaceutical Digital Temperature And Humidity Co... Grandado Temperature And Humidity In Pharmacy the world health organization states that the ideal relative humidity (rh) for pharmaceutical production environments should be. storage unit temperature/humidity distribution the range and pattern of temperatures and/or humidity within a. the exact conditions should be defined by the product, but this is normally at 50% rh and between 15°c and 25°c for drugs kept at room.. Temperature And Humidity In Pharmacy.